Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Green Synthesis of Copper Nanoparticles using Foeniculum Vulgare Seed Extract and Evaluation of their Biological Activities
Authors: Momal Saleem, Sadaf Noor, Syed Tahira Qousain Naqvi, Syed Aun Muhammad
DOI Link: https://doi.org/10.22214/ijraset.2024.57654
Certificate: View Certificate
Abstract
Copper nanoparticles are gaining popularity due to their multifarious properties and use in medicine, industry, and research. An extract of Foeniculum vulgare seed is used to green synthesize copper nanoparticles. The produced NPs were characterized by UV-visible spectroscopy, FTIR, X-ray Diffraction, and scanning electron microscopy. The absorption spectra at 570nm indicated the reduction of copper metal and the effective production of CuNPs. Its absorbance peak at 3345.3cm-1 and 1638.2cm-1 shows the existence of the N-H group and C=C stretching, as well as secondary amines (-NH-). CuNPs with a size range of 84-140nm were found to be spherical in SEM at 50kX magnification. XRD micrograph revealed a face-centered cubic structure with 19.97nm crystalline size. CuNPs showed antibacterial activity against Staphylococcus aureus ATCC25923, Salmonella enterica ATCC14028, and Pseudomonas aeruginosa ATCC27853, with ZOI ranging from 22 to 39mm. The results of the DPPH assay showed that CuNPs exhibit promising antioxidant properties, with an IC50 of 14.372 µg/ml. CuNPs had IC50 values of 4.341µg/ml for alpha-amylase and 3.37µg/ml for alpha-glucosidase, respectively. The IC50 of CuNPs from fennel seed extract was 4.1µg/ml, showing that CuNPs have anti-hyperlipidemic potential and can be utilized to treat hyperglycemia. This study found that green-synthesized CuNPs have numerous therapeutic applications in the medical and biotechnological domains.
Introduction
I. INTRODUCTION
When we talk about "Nanotechnology," we're talking about anything with a diameter of ten to one hundred nanometre or less. Small or dwarf is what the word "Nano" refers to when it is translated from Greek. Biomedical and biotechnology have never had a field as bright as Nanotechnology in today's world of cutting-edge science and technology. The high surface-to-volume ratio of nanoparticles makes them stand out. Nanoparticle stability and catalytic performance benefit from this property [1]. A material's structural and compositional properties differ from its bulk analogues at the nanoscale. Nanoparticles can be distinguished by several properties, including stability, diameter, volume, electric, magnetic, and catalytic activity [2]. Nanoparticles are the most advanced tool, with roots in almost every aspect of life [3]. It has a wide range of diagnostic and therapeutic applications. A nanomaterial is a small object with a diameter of 10-9. Until now, various metals such as silver, zinc, copper, selenium, and magnesium have been used to make nanomaterials [4]. Nanoparticles are gaining popularity in a variety of fields, including medicine, industry, and engineering, due to their unique properties. To prepare Nano-scale materials, a range of methodologies and mediums could be used, which would include lipids, polymeric substances, and multiple peptide proteins [5].
Nanoparticles are classified into a variety of categories based on the fabrication material, methodology, and other considerations, but metallic nanoparticles are the most alluring. Copper NPs, among all metals, have piqued interest for a long time due to their medicinal value. Their mesmerizing characteristics and applications in almost every aspect of human life laid the groundwork for the tech-savvy world. They're important in the fields of environmental deterioration, along with agricultural production, and bioengineering. Copper's biocidal qualities have been recognized for decades [6].
Hyperlipidaemia is a health problem that is the major cause of fatal deaths, especially among the elderly. It is a health issue in which the level of lipids or cholesterol in the blood exceeds the normal range. This elevated level of lipid in plasma is the primary factor of coronary vessel blockade resulting in cardiovascular ailments [7]. Furthermore, highly developed nanotechnology utilized NPs as the most effective drug carrier for target delivery having a great deal of potential for boosting the drug's bioavailability. NPs are composed of extracts from numerous therapeutic plant organs that can be used for treatment [8]. The NPs also act as effective antibacterial, antioxidant, and antidiabetic agents.
In the current study, the Foeniculum vulgare has been utilized for the CuNPs synthesis. Fennel seed is an outstanding treatment for reducing gastrointestinal problems. Phytoconstituents found in fennel seed make it a great antimicrobial, antispasmodic, antiflatulent, and antioxidant agent, as per various in vitro and in vivo studies. [9]. Nanoparticles have achieved this status as a result of their extraordinary and distinguishable physicochemical and surface properties. Characterization of nanoparticles is required to assess the features and functions associated with these qualities. Qualitative characterization of biosynthesized nanoparticles can be done through, a variety of techniques that are readily accessible. Ultraviolet-visible spectroscopy, Fourier Transmission Infrared Spectroscopy (FTIR), Scanning Electron Microscopy (SEM), Transmission Electron Microscopy (TEM), Dynamic Light Scattering, and Zeta Sizer are among the techniques used.
II. MATERIALS AND METHODS
Copper nitrate trihydrate salt along with other reagents was procured from Sigma Aldrich (St. Louis, MO, USA). Fennel seeds were purchased from a local departmental store.
A. Preparation of Plant Extract
Fennel seed extract was prepared by firstly washing them thoroughly 2-3 times by using tap water followed by distilled distilled water to eliminate any sort of dirt or contaminants. After washing came the boiling step for that 7g fennel seeds were boiled in 500ml of distilled water for 20 minutes at a temperature of 80?C. Later on, the filtrate was obtained by using Whatmann filter paper of pore size 125mm. The filtrate was stored at 4oC for later biomedical assay analysis [10].
B. Biosynthesis of CuNPs
Different concentrations of copper nitrate salt were prepared from the stock solution and then mixed with the fennel seed extract. The different ratios in which the solution mixture was prepared were 1:1, 1:3, 1:5, and 1:9. After the preparation of the reaction mixture of different ratios they were incubated for 24h at 37?C and after that, they were kept at room temperature for further biomedical assays [11].
C. Ultraviolet-Visible Spectrophotometer
The absorbance of biosynthesized CuNPs was analysed by adjusting the Ultraviolet-visible spectrophotometer (pg instruments T80) at the range of 300-600nm [12]
D. Fourier Transform Infrared Spectroscopy (FTIR)
Fourier Transform Infrared Spectroscopy is one of the primary protocols to analyse the attached functional groups to the synthesized nanoparticles. For this purpose, CARY 630 FTIR was used the wavelength of which was adjusted in the range limit of 650-4000 cm-1 to evaluate the nanoparticles sample [13].
E. Scanning Electron Microscopy (SEM)
The analysis of morphological properties along with the size range of CuNPs was done by Scanning Electron microscopy (SEM) (JSM-6380. SEM) at 30kv and micrographs were taken at different magnifications [14].
F. X-ray Diffraction (XRD) analysis
To examine the crystallinity or amorphic nature of the CuNPs the XRD analysis was opted. This technique gives information on whether the biosynthesized CuNPs are crystalline or amorphous along with the crystal size and shape. In XRD analysis X-rays were bombarded on the sample and a band was generated by a diffractometer [15].
G. Antibacterial activity of CuNPs
To analyse whether the biosynthesized CuNPs carry antibacterial potency they were tested against both Gram-negative and Gram-positive bacterial strains including Escherichia coli (E. coli ATCC 25922 TM), Salmonella enterica (S. enterica ATCC 14028), Klebsiella pneumoniae (K. pneuoniae ATCC 13883), Staphylococcus aureus (S. aureus ATCC 25923), Micrococcus luteus (M. luteus ATCC 10240), and Bacillus subtilis (B. subtilis ATCC 6633). The inoculums of these strains were taken in nutrient broth. The agar well method was opted to examine the antibacterial potential of the CuNPs [16]. The prepared wells in the plate were labeled and filled with 100µl of synthesized CuNPs, fennel seed extract as a negative control, and ciprofloxacin as a standard drug [17]. After filling the wells, the Petri plates were kept at 40C for 4-5 minutes for the proper dissemination of samples and then incubated at 37?C for 24 hours after that the ZOI were analysed in the plates.
H. Anti-hyperlipidemic Activity
To analyse the antihyperlipidemic capability of CuNPs the HMG-CoA reductase (HMGR) assay kit (SIGMA-ALDRICH (Catalog Number CS1090) was used. To melt the HMGR kit solutions they were thawed. The solutions were kept on ice to avoid their degradation. The enzyme HMGR, as well as the drug pravastatin, should not be present in the blank solution. For the preparation of the standard, the reaction mixture included 2.5µl of pravastatin drug. The activity of the CuNPs was estimated by the addition of 10µl reconstituted NADPH with 30µl of substrate solution HMG-CoA and 2.5µl of (HMGR) enzyme solution was reaction and inhibitory samples. Within 60 minutes the assay should be completed to avoid the degradation of the reagents of the kit. So in the end to evaluate the result the wavelength of the spectrophotometer was adjusted at 340nm at a temperature of 37?C. After a 5-second time interval, the absorption of samples was assessed, and the same procedure was repeated for 5 minutes.

I. Anti-diabetic Activity of Biosynthesized CuNPs
In vitro alpha-amylase and alpha-glucosidase assays were being used to ascertain the anti-diabetic potential of synthesized copper nanoparticles. To perform the assay, the dilutions of the CuNPs were prepared (100μg/ml, 120μg/ml, 140μg/ml, 160μg/ml, 180μg/ml, and 200μg/ml) and 50µl of them mixed with 100µl phosphate buffer pH 6.9. 50µl of the 1% freshly prepared alpha-amylase and given 10-minute incubation at 300C in the water bath. 50µl of the 1% of the potato starch solution was also added and again given 10-minute incubation at 37?C. for the termination of the reaction 150µl of the 1% of dinitrosalicylic acid (DNS) solution was added. The addition of this reagent resulted in the alteration of the colour of the mixture. A standard solution of the acarbose drug was also prepared. To access the activity all the solutions prepared earlier were put in the 96 well plate and the spectrum was generated at 405nm [18].
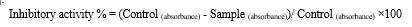
J. Anti-oxidant activity of CuNPs
The radical scavenging potential of CuNPs as antioxidant agents was assessed by 2-diphenyl-1-picrylhydrazyl DPPH assay. Different dilutions of CuNPs (as mentioned above) along with a 0.1mM solution of 1,1 Diphenyl 2- picryl hydrazyl (DPPH) in methanol were prepared [19]. 200µl of each CuNPs dilution was added to 200µl of the DPPH solution and kept under the dark conditions for half an hour at room temperature. The incubation resulted in colour change and at 517nm the absorption was analysed [20]. Ascorbic acid with dimethyl sulfoxide (DMSO) was used as standard. The percentage radical scavenging capability of CuNPs was calculated using the following equation [21].

III. RESULTS
A. Green Synthesis of CuNPs
Various concentrations i.e. (1mM-11mM) of Cu(NO3)2.3H2O salt solution was tested for nanoparticle synthesis. The concentration of 7mM exhibited a prominent indication of colour variation and NPs synthesis. Several metabolites in fennel seed extract acted as capping and reducing agents for copper salt ions. The reduction of copper ions was observed by a prominent colour transformation from brown to bluish-green after the incubation of 24 hours at 37°C. The green synthesis of CuNPs is presented in Fig. 1.
B. UV- Visible Spectrophotometry
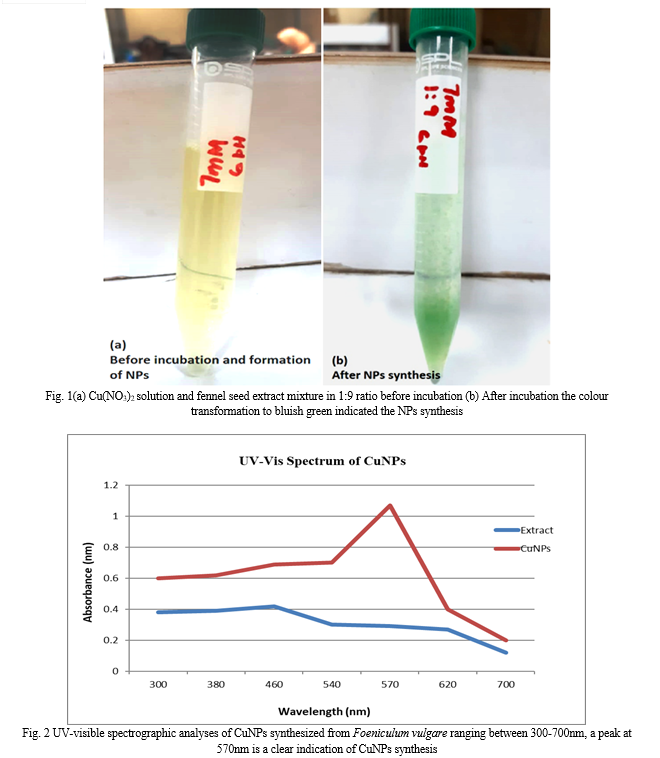
To confirm the CuNPs synthesis UV-visible spectrophotometry was opted and performed by adjusting the range of the spectrophotometer between 300-700nm. Earlier the investigations carried out by various scientists reported absorption bands for CuNPs between 500-600nm, in the case of our research a prominent band was observed at 570nm (Fig. 2). The process of Surface Plasmon Resonance and its direct relationship to the size of NPs was a major cause of this broad absorption peak, and as a result, different researchers reported different CuNP absorption peaks. Fennel seed extract did not have any band in between the range of 500-600nm.
C. Fourier-Transform Infrared Spectroscopy Analysis (FTIR)
Fennel seed extract functional groups which act as pivotal reducing and capping agents were identified via FTIR. The biosynthesized CuNPs were analyzed using FTIR in the 650-4000 cm-1 wave region. Prominent peaks of absorption at 3345.3 and 1638.2 cm-1 had been observed for CuNPs in the FTIR spectrum (Fig. 3).
The absorption band at 3345.3 cm-1 was due to secondary amines and had been a clear indication of the attachment of the N-H group to the CuNP on the FTIR spectrograph. The second peak at 1638.2 cm-1 was due to C=C stretching and CuNP stabilization because of secondary amines.
D. Scanning Electron Microscopy (SEM)
SEM was used to examine the nano morphological properties of CuNPs at 50kX magnification. Biosynthesized CuNPs have spherical aggregates with sizes ranging from 84 to 140nm, according to SEM micrographs (Fig. 4). The most common size of CuNPs reported is 500nm, indicating green synthesis of CuNPs.
E. X-ray Diffraction Analysis (XRD)
X-ray diffraction revealed the crystallinity of the biosynthesized copper nanoparticles having the cubic-centered shape of crystals. The CuNPs' crystallinity was further confirmed by their crystalline index of 53.82 percent. Origin software was used for plotting an XRD chart which was further to compare it with the standard (JCPDS 04-0836) revealing several distinct peaks at 39.69°, 47.51°, 50.90°, and 70.8660°. These peaks correspond to the planes (111), (200), and (220). Debye-equation Scherrer's D = 0.9/(cos) was used to calculate the size of the CuNPs crystal. CuNPs had an average crystalline size of 19.97nm when tested.
F. Antibacterial assay of biosynthesized CuNPs
The antibacterial capability of CuNPs was tested against six bacterial strains e.g. Escherichia coli (E. coli ATCC 25922 TM), Bacillus subtilis (B. subtilis ATCC 6633 TM), Staphylococcus aureus (S. aureus ATCC 25923), Salmonella enteric (S. enterica ATCC 14028), and Pseudomonas aeruginosa (P. aeruginosa ATCC 27853 TM). Among all the strains the CuNPs exhibited the largest inhibitory zones of 34mm and 39mm against M. leutues and S. aureus. Whereas the CuNPs exhibited a significant inhibitory zone against the remaining strains proving it to be a potential antibacterial agent.
G. Anti-hyperlipidemic activity of CuNPs
HMG-CoA reductase assay of CuNPs at concentrations (100-180μg/ml) exhibited significant potential having the IC50 value of 4.1μg/ml. Fig. 7 depicts the anti-hyperlipidemic ability of CuNPs and the standard.
H. Anti-diabetic activity of CuNPs
The results of the anti-diabetic assay of biogenic CuNPs have been indicated in Fig. 8 and 9. Alpha amylase and alpha-glucosidase assays indicated that the CuNPs had a great capability of being an effective anti-diabetic displaying the IC50 of 4.341μg/ml and 3.37μg/ml, respectively.
I. Anti-oxidant activity of CuNPs
The study of biogenic CuNPs' radical scavenging abilities divulged that they have a strong ability to scavenge the noxious oxidants found in the human body. CuNPs having concentration (10-100µg/ml) showed a repressive activity against reactive oxygen species with an IC50 value of 14.372 µg/ml (Table. 1). Ascorbic acid was used as a positive control.
IV. DISCUSSION
In this study, copper nanoparticles were synthesized using a less expensive, ecologically responsible, and time-consuming method. Copper nitrate salt (Cu(NO3)2).3H2O was added to the boiled seed extract of Foeniculum vulgare in the ratio of (1:9, 7mM). After a 24-hour incubation period at 37°C, a distinct colour transformation from light brown to bluish green was witnessed, and the utmost nanoparticles were acquired using this method. Showmya and colleagues have already designed and synthesized nanoparticles from fennel seed extract [22]. As a result, CuNPs were successfully prepared at a concentration of 7mM (9:1) in the research study, showcasing a broad range of biological activities.
Following the biosynthesis of CuNPs, the process of characterization was used to confirm their formation. The formation and surface characteristics of the nanoparticle samples were first verified using UV spectrophotometry, which confirmed the biosynthesis of CuNPs. The finest peaks had been revealed by biosynthesized CuNPs in a band range of 300-600nm. CuNPs synthesized by utilizing the fennel seed extract revealed the most prominent peak at 570nmCuNP absorption peaks at 544.89 nm were reported by Gondwal and his colleagues in a research article that contained findings that were somewhat similar to this research project [23].
FTIR analysis of CuNPs revealed two sharp peaks giving information regarding the functional groups aiding in the stabilization of CuNPs. FTIR spectrograph depicts peaks at 3345.3 and 1638.2cm-1 which signified the role of amines in stabilizing the nanoparticles due to the N-H bond and alkenes due to C=C bond attachment, Murthly et al. also reported quite a similar finding with their synthesized CuNPs by reporting FTIR spectra range between 3351 to 570cm-1 [24]. SEM images of biologically synthesized CuNPs showed the formation of spherical aggregates with a size range of 84nm-306nm and an average diameter of 84nm, with the majority of the CuNPs being 84nm in size. Palavi and his colleagues found that biosynthesized CuNPs had a size of less than 100nm [25]. The XRD analysis of CuNPs revealed the cubic-centered structure and Miller indices at (111), (200), and (220), indicating crystallinity and crystal size of 19.97nm in comparison to the standard (JCPDS 04-0836). As a result of following the standard JCPDS, the team led by Y.T. Prabhu discovered the same miller indices and crystal size (26.51nm) [26].
This study proposes a cheap, convenient, and low-impact solution to these issues. The current study aimed to synthesize CuNPs from fennel seed extract, as these NPs have antibacterial properties. CuNPs' biocidal strength was tested against gram-negative and gram-positive bacterial strains. Bacillus subtilis (B.subtilis ATCC 6633 TM), Staphylococcus aureus (S.aureus 25923), Salmonella enteric (S. enterica ATCC 14028), and Pseudomonas aeruginosa (P. aeruginosa ATCC 27853 TM) were also examined and measured after a 24-hour incubation period. M. leuteus had a ZOI of 34mm, while S. aureus had a ZOI of 39mm. Amer and his coworkers looked into the antibacterial properties of CuNPs against E. coli and S. aureus, both of which had a ZOI of 25mm [27]. As a result of these earlier studies, our green synthesized CuNPs proved to be more effective in the fight against bacterial strains. Previously, Caroling and his colleagues studied the bacteriocidal capabilities of CuNPs and the mechanism behind this biocidal activity, and they found that CuNPs can disrupt bacterial membranes, resulting in cell death, which supported our findings [28]. The hyperlipidemic potential of CuNPs was assessed by comparing them to the standard drug Pravastatin. Pravastatin had an IC50 of 4.734µg/ml while CuNPs had an IC50 of 4.1µg/ml. Our findings are effective because the lower the IC50, the more anti-hyperlipidemic effect the nanoparticles have [29]. CuNPs have the exceptional power to inhibit alpha-amylase, which is proof of their anti-diabetic capability and remedial effects. CuNPs have been shown to inhibit alpha-amylase (IC50 = 57.49µg/ml) and glucosidase (IC50 = 57.25µg/ml) by Chizoba Ekezie FG and colleagues [30]. Our study shows that CuNPs carry substantial inhibitory properties and a potential capability to treat diabetic individuals by exhibiting the IC50 value of 4.341μg/ml and 3.37μg/ml for α-amylase and α-glucosidase respectively. The scavenging ability of CuNPs synthesized from fennel seed was evaluated using the diphenyl 2-picryl hydrazyl (DPPH) assay. The reaction mixture changed color from deep purple to pale yellowish when a hydrogen atom was donated to reduce DPPH. The IC50 value of 14.372µg/ml for the green synthesized CuNPs proves them to be the best antioxidant agents. Muthuve et al. reported the antioxidant potential of copper oxide NPs synthesized from Solanum nigrum leaves, with an IC50 of 131.54µg/ml for various concentrations [31].




Conclusion
Our research found that copper nanoparticles from Foeniculum vulgare have a wide range of biomedical applications. The average crystal size was 19.97nm for face-centred cubic crystalline CuNPs. SEM revealed agglomerated spherical forms with 84nm particle size. CuNPs offer a wide range of antibacterial applications, with a ZOI of 22-39mm against Gram-positive and Gram-negative bacteria. They have outstanding and adequate anti-diabetic efficacy (IC50 = 4.341µg/ml and 21.814µg/ml), anti-oxidant potency (IC50 = 14.372µg/ml), and anti-hyperlipidemic potency (IC50 = 4.1µg/ml) compared to pravastatin. Copper nanoparticle synthesis via green method straightforward, viable, cost-effective, and one-step approach. These particles have a variety of biomedical applications and have the potential to be employed efficiently in a medication delivery system. As a result, these copper nanoparticles may be an effective choice for the development of novel anithyperlipidemic, antidiabetic, and antibacterial agents.
References
[1] Bhagat M., Anand R., Sharma P., Rajput P., Sharma N., and Singh K. 2021. Multifunctional Copper Nanoparticles: Synthesis and Applications. ECS Journal of Solid State Science and Technology, vol 10, pages 1-11. doi: 10.1149/ 2162-8777/ac07f8. [2] Bhattacharya R. and Mukherjee P. 2008. Biological properties of “naked” metal nanoparticles. Advanced Drug Delivery Reviews, vol 60, pages 1289-1306. doi: https://doi.org/10.1016/j.addr.2008.03.013. [3] Salata O.V. 2004. Applications of nanoparticles in biology and medicine. Journal of Nanobiotechnology, vol. 2, pages 3. doi: https://doi.org/10.1186/1477-3155-2-3. [4] Panigrahi S., Kundu S., Ghosh S., Nath S., and Pal T. 2004. General method of synthesis for metal nanoparticles. Journal of Nanoparticle Research, vol. 6, pages 411-414. doi: https://doi.org/10.1007/s11051-004-6575-2. [5] Jeevanandam J., Barhoum A., Chan Y.S., Dufresne A., and Danquah M.K. 2018. Review on nanoparticles and nanostructured materials: history, sources, toxicity and regulations. Beilstein Journal of Nanotechnology, vol 9, page 1050-1074. doi: https://doi.org/10.3762/bjnano.9.98. [6] Murthy H.A., Prakash C., Abebe B., and Shantaveerayya K. Green Synthesis of Cu/CuO and Ag2O Nanomaterials for Multifunctional Applications. Current Research in Science and Technology, vol 4, Page 113-131, doi: http://dx.doi.org/10.13005/msri/150311. [7] Gupta N., Sharma N., Mathur S.K., Chandra R., and Nimesh S. 2018. Advancement in nanotechnology-based approaches for the treatment and diagnosis of hypercholesterolemia. Artificial Cells, Nanomedicine and Biotechnology, vol 46, pages 188-197. doi: https://doi.org/10.1080/21691401.2017.1417863. [8] Sharma K., Kumar K., and Mishra N. 2016. Nanoparticulate carrier system: a novel treatment approach for hyperlipidemia. Journal of Drug Delivery, vol 23, pages 684-699. doi: https://doi.org/10.3109/10717544.2014.920937. [9] Akaberi M., Iranshahy M., and Iranshahi M. 2015. Review of the traditional uses, phytochemistry, pharmacology and toxicology of giant fennel (Ferula communis L. subsp. communis). Iranian Journal of Basic Medical Sciences, vol 18, pages 1050. 1050. [10] Choudhary M.K., Kataria J., and Sharma S. 2017. A biomimetic synthesis of stable gold nanoparticles derived from aqueous extract of Foeniculum vulgare seeds and evaluation of their catalytic activity. Applied Nanoscience, vol 7, pages 439-447. doi: https://doi.org/10.1007/s13204-017-0589-4. [11] Amer M.W. and Awwad A.M. 2021. Green synthesis of copper nanoparticles by Citrus limon fruits extract, characterization and antibacterial activity. International Journal of Chemistry, vol 7, pages 1-8. doi: https://doi.org/10.31219/osf.io/76k8r. [12] Thiruvengadam M. et al. 2019. Synthesis, characterization and pharmacological potential of green synthesized copper nanoparticles. Bioprocess and Biosystems Engineering, vol 42, pages 1769-1777. doi: https://doi.org/10.1007/s00449-019-02173-y. [13] Amaliyah S., Pangesti D.P., Masruri M., Sabarudin A., and Sumitro S.B. 2020. Green synthesis and characterization of copper nanoparticles using Piper retrofractum Vahl extract as bioreductor and capping agent. Heliyon, vol 6, pages 12. doi: https://doi.org/10.1016/j.heliyon.2020.e04636. [14] Anju S.S., Dhanetia H.R., and Sharma A. 2020. GREEN SYNTHESIS OF COPPER NANOPARTICLES USING Holoptelea integrifolia FRUIT EXTRACT. RAS?YAN Journal of Chemistry, vol 13, pages 2664-2671. doi: http://dx.doi.org/10.31788/ RJC.2020.1346306. [15] Patel B., Channiwala M., Chaudhari S., and Mandot A. 2016. Biosynthesis of copper nanoparticles; its characterization and efficacy against human pathogenic bacterium. Journal of Environmental Chemical Engineering, vol 4, pages 2163-2169. doi: http://dx.doi.org/10.31788/ RJC.2020.1346306 [16] Dwarakanath P., Rajakumari K., and Thiruchelvi R. 2021. Isolation and Characterization of Glucanase producing bacteria from Forest Tree Litter. Research Journal of Pharmacy and Technology, vol 14, pages 916-920. doi: DOI: 10.5958/0974-360X.2021.00163.3. [17] Manjunatha K., Bhat R.S., Shashidhara A., Kumar H.A., and Nagashree S. 2021. Antimicrobial and Nonlinear Optical Studies of Copper Oxide Nanoparticles. Journal of Electronic Materials, pages 1-7. doi: https://doi.org/10.1007/s11664-021-08838-3. [18] Sagbo I.J., van de Venter M., Koekemoer T., and Bradley G. 2018. In vitro antidiabetic activity and mechanism of action of Brachylaena elliptica (Thunb.) DC. Evidence-Based Complementary and Alternative Medicine, vol 2018, pages 13. doi: https://doi.org/10.1155/2018/4170372. [19] Das D., Nath B.C., Phukon P., and Dolui S.K. 2013. Synthesis of ZnO nanoparticles and evaluation of antioxidant and cytotoxic activity. Colloids and Surfaces B: Biointerfaces, vol 111, pages 556-560. doi: https://doi.org/10.1016/j.colsurfb.2013.06.041. [20] Muthuvel A., Jothibas M., and Manoharan C. 2020. Synthesis of copper oxide nanoparticles by chemical and biogenic methods: photocatalytic degradation and in vitro antioxidant activity. Nanotechnology for Environmental Engineering, vol 5, 1-19. doi: https://doi.org/10.1007/s41204-020-00078-w. [21] Wu S., Rajeshkumar S., Madasamy M., and Mahendran V. 2020. Green synthesis of copper nanoparticles using Cissus vitiginea and its antioxidant and antibacterial activity against urinary tract infection pathogens. Artificial Cells, Nanomedicine, and Biotechnology, vol 48, pages 1153-1158. doi: https://doi.org/10.1080/21691401.2020.1817053. [22] Showmya J. et al. 2012. Rapid green synthesis of silver nanoparticles using seed extract of Foeniculum vulgare and screening of its antibacterial activity. Plant Cell Biotechnology and Molecular Biology, vol 13, pages 31-38. [23] Gondwal M. and Joshi nee Pant G. 2018. Synthesis and catalytic and biological activities of silver and copper nanoparticles using Cassia occidentalis. International Journal of Biomaterials, vol 2018, pages 11. doi: https://dx.doi.org/10.1155%2F2018%2F6735426. [24] Murthy H., Desalegn T., Kassa M., Abebe B., and Assefa T. 2020. Synthesis of green copper nanoparticles using medicinal plant hagenia abyssinica (Brace) JF. Gmel. leaf extract: Antimicrobial properties. Journal of Nanomaterial, vol 2020, pages 12. doi: https://doi.org/10.1155/2020/3924081. [25] Chauhan P.S., Shrivastava V., and Tomar R.S. 2018. Biofabrication of Copper Nanoparticles: A Next-generation Antibacterial Agent Against Wound-associated Pathogens. Turkish Journal of Pharmaceutical Sciences, vol. 15, pages 238. doi: https://dx.doi.org/10.4274%2Ftjps.52724. [26] Prabhu Y., Rao K.V., Sai V.S., and Pavani T. 2017. A facile biosynthesis of copper nanoparticles: a micro-structural and antibacterial activity investigation. Journal of Saudi Chemical Society, vol 21, pages 180-185. doi: https://doi.org/10.1016/j.jscs.2015.04.002. [27] Amer M.W. and Awwad A.M. 2021. Green synthesis of copper nanoparticles by Citrus limon fruits extract, characterization and antibacterial activity. Chemistry International, vol 7, pages 1-8. doi: https://doi.org/10.5281/zenodo.4017993. [28] Caroling G., Priyadharshini M.N., Vinodhini E., Ranjitham A.M., and Shanthi P. 2015. Biosynthesis of copper nanoparticles using aqueous guava extract-characterisation and study of antibacterial effect.s International Journal of Pharmacy and Biological Sciences, vol 5, pages 25-43. [29] Ginting B., Maulana I., and Karnila I. 2020. Biosynthesis copper nanoparticles using Blumea balsamifera leaf extracts: characterization of its antioxidant and cytotoxicity activities. Surfaces and Interfaces, vol 21, pages 27. doi: https://doi.org/10.1016/j.surfin.2020.100799. [30] Chizoba Ekezie F., Suneetha J., and Uma Maheswari K. 2016. In Vitro Carbohydrate Digestibility of Copper Nanoparticulated Bitter Gourd Extract. Journal of Nutrition & Food Sciences, vol 6, pages 482. doi: doi:10.4172/2155-9600.1000482. [31] Muthuvel A., Jothibas M., and Manoharan C. 2020. Synthesis of copper oxide nanoparticles by chemical and biogenic methods: photocatalytic degradation and in vitro antioxidant activity. Nanotechnology for Environmental Engineering, vol 5, pages 14. doi: https://doi.org/10.1007/s41204-020-00078-w.
Copyright
Copyright © 2024 Momal Saleem, Sadaf Noor, Syed Tahira Qousain Naqvi, Syed Aun Muhammad. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET57654
Publish Date : 2023-12-20
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

